

The first collection included 48 meta-analyses (421 trials) published in the 10 leading journals of each medical subject category of the Journal Citation Reports during two periods: between July 2008 and January 2009 and between January and June 2010 or in issue 4 of the Cochrane Database of Systematic Reviews in 2008. This study combined data from two independent collections of meta-analyses of randomised controlled trials assessing therapeutic interventions with binary outcomes.
#Issues with smalland large sample size trial
In this study, we assessed the influence of trial sample size on treatment effect estimates in a large collection of meta-analyses of various medical conditions and interventions. Therefore, a trial of 1000 patients can be considered large for certain medical conditions and small for others. 7 For binary outcomes, the required sample size depends on the magnitude of treatment effect as well as the number of events and frequency of the medical condition. The concept of a single threshold to distinguish small trials from large trials, whatever the medical area or intervention being tested, is not straightforward. 3 4 5 A study based on a collection of meta-analyses in osteoarthritis showed that trials including fewer than 100 patients per arm yielded, on average, greater treatment effect estimates than did larger trials.
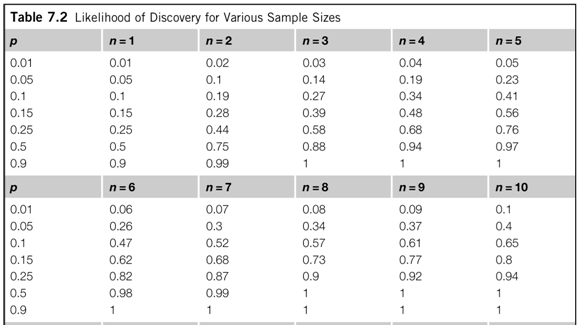
Our knowledge about the influence of trial sample size on treatment effect estimates is based on the small study effect-the tendency for small trials to report greater treatment benefits than large trials in the same meta-analysis. For example, a meta-analysis in cardiology 2 included trials with sizes ranging from 62 patients to 45 852 patients. Sample size varies greatly among trials, ranging from tens of patients to thousands of patients, 1 even within a meta-analysis investigating the same question.

Compared with trials of 1000 patients or more, treatment effects were, on average, 48% larger in trials with fewer than 50 patients (0.52, 0.41 to 0.66) and 10% larger in trials with 500-999 patients (0.90, 0.82 to 1.00).Ĭonclusions Treatment effect estimates differed within meta-analyses solely based on trial sample size, with stronger effect estimates seen in small to moderately sized trials than in the largest trials. Similar results were obtained when comparing treatment effect estimates between different size groups. Compared with quarter 4 (which included the largest trials), treatment effects were, on average, 32% larger in trials in quarter 1 (which included the smallest trials ratio of odds ratios 0.68, 95% confidence interval 0.57 to 0.82), 17% larger in trials in quarter 2 (0.83, 0.75 to 0.91), and 12% larger in trials in quarter 3 (0.88, 0.82 to 0.95). Results Treatment effect estimates were significantly larger in smaller trials, regardless of sample size. Treatment effects were compared within each meta-analysis between quarters or between size groups by average ratios of odds ratios (where a ratio of odds ratios less than 1 indicates larger effects in smaller trials). Objective To assess the influence of trial sample size on treatment effect estimates within meta-analyses.ĭata sources 93 meta-analyses (735 randomised controlled trials) assessing therapeutic interventions with binary outcomes, published in the 10 leading journals of each medical subject category of the Journal Citation Reports or in the Cochrane Database of Systematic Reviews.ĭata extraction Sample size, outcome data, and risk of bias extracted from each trial.ĭata synthesis Trials within each meta-analysis were sorted by their sample size: using quarters within each meta-analysis (from quarter 1 with 25% of the smallest trials, to quarter 4 with 25% of the largest trials), and using size groups across meta-analyses (ranging from <50 to ≥1000 patients).


 0 kommentar(er)
0 kommentar(er)
